Hydrochloric Acid 28%
Structure and reactions
Hydrochloric acid is the salt of the protonated water and chloride. Its ions are often written as H3O+ Cl−,[25] although the cation is in fact often bonded to other water molecules. A combined IR, Raman, X-ray, and neutron diffraction study of concentrated hydrochloric acid revealed that the primary form of H+(aq) in these solutions is H5O2+, which, along with the chloride anion, is hydrogen-bonded to neighboring water molecules in several ways.[26] (See Hydronium for further discussion of this issue.)
Acidity
As a strong acid, hydrogen chloride has a large Ka. Theoretical estimates suggest that the pKa of hydrogen chloride is −5.9.[5] However, it is important to distinguish between hydrogen chloride gas and hydrochloric acid. Due to the leveling effect, except when highly concentrated and behavior deviates from ideality, hydrochloric acid (aqueous HCl) is only as acidic as the strongest proton donor available in water, the aquated proton (popularly known as “hydronium ion”). When chloride salts such as NaCl are added to aqueous HCl, they have only a minor effect on pH, indicating that Cl− is a very weak conjugate base and that HCl is fully dissociated. Dilute solutions of HCl have a pH close to that predicted by assuming full dissociation into hydrated H+ and Cl−.[27]
Physical properties
|
Mass |
||||||||||
|
kg HCl/kg |
kg HCl/m3 |
kg/L |
mol/L |
mPa·s |
kJ/(kg·K) |
kPa |
°C |
°C |
||
|
10% |
104.80 |
6.6 |
1.048 |
2.87 |
−0.5 |
1.16 |
3.47 |
1.95 |
103 |
−18 |
|
20% |
219.60 |
13 |
1.098 |
6.02 |
−0.8 |
1.37 |
2.99 |
1.40 |
108 |
−59 |
|
30% |
344.70 |
19 |
1.149 |
9.45 |
−1.0 |
1.70 |
2.60 |
2.13 |
90 |
−52 |
|
32% |
370.88 |
20 |
1.159 |
10.17 |
−1.0 |
1.80 |
2.55 |
3.73 |
84 |
−43 |
|
34% |
397.46 |
21 |
1.169 |
10.90 |
−1.0 |
1.90 |
2.50 |
7.24 |
71 |
−36 |
|
36% |
424.44 |
22 |
1.179 |
11.81 |
−1.1 |
1.99 |
2.46 |
14.5 |
61 |
−30 |
|
38% |
451.82 |
23 |
1.189 |
12.39 |
−1.1 |
2.10 |
2.43 |
28.3 |
48 |
−26 |
|
The reference temperature and pressure for the above table are 20 °C and 1 atmosphere (101.325 kPa). |
||||||||||
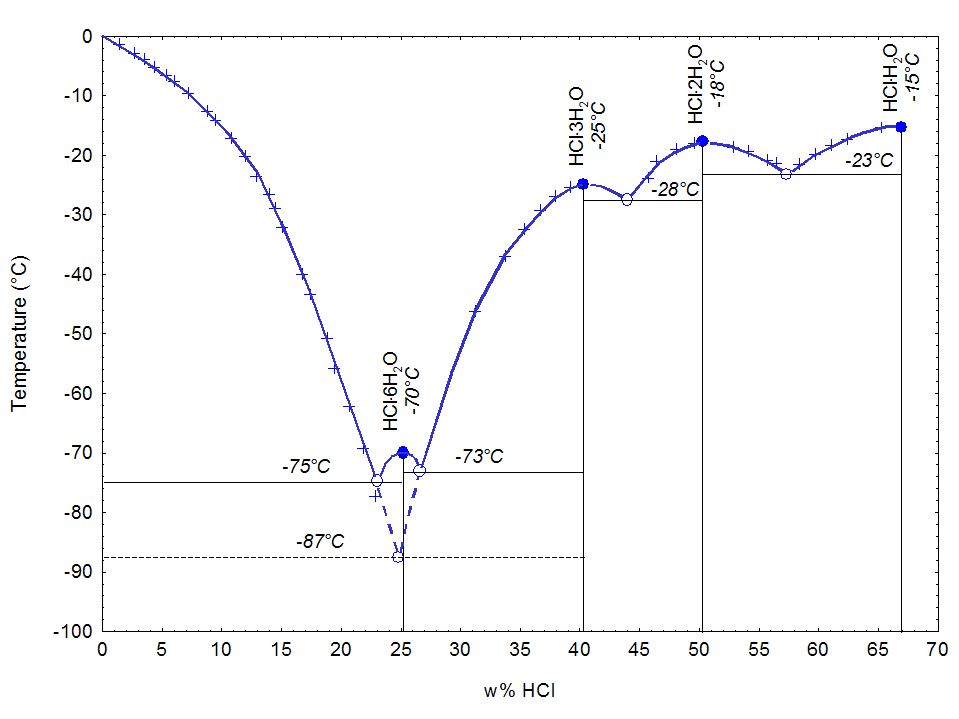
Melting temperature as a function of HCl concentration in water[28][29]
Physical properties of hydrochloric acid, such as boiling and melting points, density, and pH, depend on the concentration or molarity of HCl in the aqueous solution. They range from those of water at very low concentrations approaching 0% HCl to values for fuming hydrochloric acid at over 40% HCl.[30][31][32]
Hydrochloric acid as the binary (two-component) mixture of HCl and H2O has a constant-boiling azeotrope at 20.2% HCl and 108.6 °C (227 °F). There are four constant-crystallization eutectic points for hydrochloric acid, between the crystalform of [H3O]Cl (68% HCl), [H5O2]Cl (51% HCl), [H7O3]Cl (41% HCl), [H3O]Cl·5H2O (25% HCl), and ice (0% HCl). There is also a metastable eutectic point at 24.8% between ice and the [H7O3]Cl crystallization.[32] They are all Hydronium salts.
This page uses material from the related Wikipedia article which is released under the Creative Commons Attribution-Share-Alike License 3.0.
